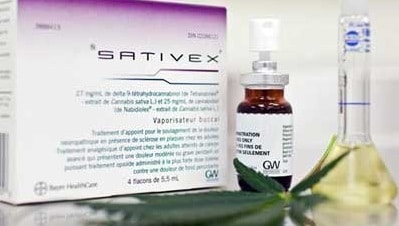
Sativex is unique in that it is the first pharmaceutical drug made from the whole cannabis plant. It contains real cannabinoids (active molecules of cannabis) and not synthetic elements.
If other medications comparable to Sativex exist and it is sometimes similar to cannabis tinctures or oils, it may be relevant to ask the following questions:
-
-
- How is it different from other cannabinoid-containing drugs?
- Can it be compared to medical cannabis?
- Is it legal in France in 2021?
- Where can I get some?
- Is it reimbursed by social security?
- What is its price, its mode of action?
-
This medicine raises legitimate questions since the stigmatization of cannabis as a drug is still relevant at the same time that the “big pharmaceutical industry” is highlighting the real capacities of this plant to relieve patients. In particular, with the multiplication of
CBD shops
. The use of Sativex appears to be an important step for the advancement of medical cannabis in Europe and worldwide.
Sativex, a separate drug
Sativex (or nabiximols) was created by GW Pharmaceuticals in 1998. At present, it is available in no less than 30 countries.
It is obtained from the whole hemp plant. Specifically, it is a concentrate of two cannabinoids unique to cannabis:
-
- THC or tetrahydrocannabinol
CBD
or cannabidiol
This cannabis concentrate with a ratio close to 1:1 (THC:CBD) is consumed in the form of a mouth spray.
Its use is recommended when reference treatments administered to patients with moderate or severe spasticity due to multiple sclerosis (MS) do not provide sufficient relief.
Indeed, we note an effectiveness of this treatment with cannabinoids in approximately 10% of patients insufficiently relieved by a referential antispastic treatment.
The Sativex spray, composition, concentration and use
Composition and concentration
As mentioned above, the Sativex spray manufactured by the British company GW Pharmaceuticals contains a 1:1 ratio of THC and CBD. These 2 cannabinoids originally present in the hemp plant represent the 2 main active ingredients of this very special drug. However, such a balance is difficult to achieve since cannabis naturally contains a much higher THC content than CBD.
Each spray (100 µL) of this spray contains :
- 2.7 mg of tetrahydrocannabinol (THC)
- 2.5 mg of cannabidiol (CBD)
- Non-medicinal ingredients include: ethanol, propylene glycol and peppermint oil.
Use and dosage
The Sativex spray is sprayed directly into the mouth, sublingual or inside the cheek.
The dosage of this medication depends on the patient’s medical condition and sensitivity to this treatment. The right dose is when effective relief is achieved without significant side effects.
For most subjects, 4 to 8 daily doses are sufficient. However, for an adult new to treatment, the general dosage is:
- a spray twice a day, the first day. The effects are supposed to be felt after 30 minutes.
- From the 2nd day onwards, it may be appropriate to do 1 additional spray every 24 hours. However, you should not exceed 12 sprays per day. In addition, these sprays should be spaced at regular intervals.
Marketing authorization, price and reimbursement of Sativex in France
On January 8, 2014, Sativex obtained its marketing authorization (MA) in France. This medication must be prescribed by a neurologist and a hospital rehabilitation specialist. And only for certain patients with multiple sclerosis to relieve severe contractures (spasticity) that are resistant to other usual treatments. It is the laboratory Almirall which is entrusted with the French marketing of this drug.
This drug is already sold in many European countries (Netherlands, Spain, Italy, Germany, …), in Canada, Australia and in many American states. But, in France, despite its MA obtained in 2014, the sale of Sativex on French soil is still experiencing a blockage. Indeed, the company Almirall, the Economic Committee for Health Products (CEPS) and the laboratory GW Pharmaceuticals, manufacturer of Sativex, are unable to reach an agreement in their negotiations:
- Almirall proposes to sell Sativex 350 euros per box
- The CEPS, which is the interministerial body that sets the prices of drugs and medical devices covered by compulsory health insurance, offers a rate not exceeding 90 euros.
- The GW Pharmaceuticals laboratory has made an effort by lowering its prices to 320 euros (instead of 440 euros on average in Europe)
Despite a favorable opinion in October 2014 from the Haute Autorité de Santé (HAS) to grant a 15% reimbursement for this treatment, in 2016, a new marketing request remains unfulfilled because of the CESP Committee which is still blocking.
Can we buy Sativex in a pharmacy in France?
As said above, Sativex is a product with its AMM on the French territory. However, due to pricing blockages, it is still not available in our pharmacies.
Its price and the fact that it contains natural THC and CBD differentiates it from other pharmaceutical “cannabinoids” such as Marinol. To name but one!
But, the decree of October 9, 2020 published in the official journal (1) suggests a crucial advance in favor of therapeutic cannabis. And, thus, a step towards the marketing of Sativex in pharmacies. This decree authorizes an experimental use of medical cannabis on 3000 patients over a period of 2 years. The objective is to evaluate, under real conditions, the recommendations of the Commission de santé, de sécurité et des conditions de travail (CSST):
- medicines based on dried hemp flowers and full spectrum extracts (fullspectum oil)
- products with immediate effect (sublingual and inhaled products based on oil and dried flowers for vaporization)
- long-acting forms (oral products in the form of drinkable solution or hemp oil capsules).
L
n March 26, 2021, theFrance has started this experimentation of therapeutic cannabis. It involves 3,000 patients with serious diseases such as:
- epilepsy, neuropathic pain, multiple sclerosis…
On June 9, 2021, the French National Agency for the Safety of Medicines and Health Products (ANSM) announced the establishment of a new temporary scientific committee. It is composed of 13 members responsible for the follow-up of this experimentation in order to :
- to observe the good progress of this experimentation
- to be able to propose adjustments in dosage and implementation.
Medical cannabis is already prescribed and sold in many European countries and around the world. Sativex has already obtained its MA in France. We can hope that this experimentation of the therapeutic cannabis will come then definitively to unblock the administrative and financial situation of the treatments with cannabinoids. In particular, those naturally present in the hemp plant. And, will positively respond to the French pharmaceutical standards.
Sativex, Marinol and Epidiolex, drugs that are the talk of the town
Currently, there are 3 cannabinoid-based medications that have received a temporary use authorization from the ANSM or a MA. Each of them has a link to cannabis and only a doctor can prescribe them. On the other hand, they differ in their composition and, therefore, in their use:
-
- Sativex: a drug with equivalent concentrations of CBD and THC molecules. Particularly interesting product for moderate or severe forms of spasticity in patients with multiple sclerosis. It is not marketed to the great displeasure of patients because its price is considered too high to date.
- Marinol is considered as an analgesic, an anti-vomiting agent and an excellent ally for people suffering from illnesses causing a strong decrease in appetite. Its active ingredient is dronabinol which is a synthetic cannabinoid mimicking the activity of THC.
-
- Epidiolex is the first cannabis-based medicine for certain epilepsies (
Dravet syndrome
and Lennox-Gastaut syndrome). Like Sativex, it is composed of real cannabinoids but does not contain THC. Its active compound is cannabidiol (CBD).
- Epidiolex is the first cannabis-based medicine for certain epilepsies (
To conclude on Sativex, this cannabis medicine
By its composition (THC and CBD) Sativex is very similar to the cannabis tinctures, sublingual sprays and
fullspectrum hemp oils
that can be found in dispensaries in countries open to medical cannabis. Like medical cannabis, which meets pharmaceutical standards, it is prescribed by doctors and dispensed by pharmacists. However, it is strongly distinguished by its manufacturing method, which is restricted to the British pharmaceutical company GW Pharmaceuticals.
FAQ: CBD and Sativex
[sp_easyaccordion id=”9740″]
References
1 Official Journal Decree of October 9, 2020: https://www.legifrance.gouv.fr/download/pdf?id=zyKaQMl-O9XMuCnn2OUL_lbzpqBy_eO97Xv1twZ4Wyg=